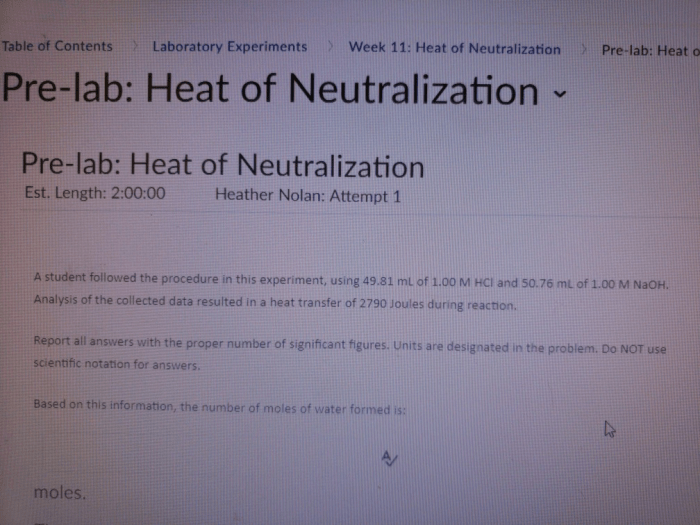
Unveiling the mysteries of heat of neutralization pre lab answers, this comprehensive guide embarks on an academic odyssey, delving into the depths of experimental setups, procedures, and data analysis, culminating in a profound understanding of this intriguing chemical phenomenon.
In this pre lab exploration, we will dissect the concept of heat of neutralization, unraveling its significance and purpose. By meticulously following the step-by-step guide, you will master the art of experimental setup, ensuring accurate data collection and analysis.
Heat of Neutralization

Neutralization is a chemical reaction in which an acid and a base react to form salt and water. The heat of neutralization is the amount of heat released or absorbed during this reaction. It is an important thermodynamic property that can provide insights into the strength of acids and bases.
A pre-lab for heat of neutralization experiments is essential for planning and understanding the experiment. It helps students prepare for the lab, understand the concepts involved, and minimize errors during the experiment.
Experimental Setup
The experimental setup for a heat of neutralization experiment typically involves the following steps:
- Calibrate a calorimeter to measure the temperature change.
- Prepare solutions of known concentrations of the acid and base.
- Measure the initial temperature of the calorimeter and the solutions.
- Add the acid solution to the base solution in the calorimeter.
- Stir the solution and record the temperature change.
A diagram of the experimental apparatus is shown below:

Procedure, Heat of neutralization pre lab answers
The procedure for conducting a heat of neutralization experiment is as follows:
- Calibrate the calorimeter by adding a known amount of heat and measuring the temperature change.
- Prepare solutions of known concentrations of the acid and base.
- Measure the initial temperature of the calorimeter and the solutions.
- Add the acid solution to the base solution in the calorimeter.
- Stir the solution and record the temperature change.
- Repeat steps 4-5 for different concentrations of the acid and base.
Data Analysis
The heat of neutralization can be calculated using the following equation:
Q = mcΔt
where:
- Q is the heat of neutralization (in Joules)
- m is the mass of the solution (in grams)
- c is the specific heat capacity of the solution (in J/g°C)
- Δt is the change in temperature (in °C)
The enthalpy change of the reaction can be determined by plotting the heat of neutralization against the concentration of the acid or base. The slope of the graph will give the enthalpy change.
Discussion
The heat of neutralization is a measure of the strength of an acid or base. A strong acid or base will release more heat when neutralized than a weak acid or base. The heat of neutralization can also be used to determine the equilibrium constant for a neutralization reaction.
There are several factors that can affect the heat of neutralization, including:
- The concentration of the acid and base
- The temperature of the reaction
- The presence of other ions in the solution
It is important to be aware of these factors when designing and conducting a heat of neutralization experiment.
Potential sources of error in a heat of neutralization experiment include:
- Inaccurate measurement of the temperature change
- Incomplete reaction between the acid and base
- Heat loss to the surroundings
By carefully controlling the experimental conditions and taking appropriate precautions, these sources of error can be minimized.
Key Questions Answered: Heat Of Neutralization Pre Lab Answers
What is the purpose of a pre lab for heat of neutralization experiments?
A pre lab serves as a preparatory guide, providing a detailed overview of the experiment’s objectives, materials, and procedures. It ensures a thorough understanding of the experiment, minimizing errors and maximizing learning outcomes.
How do I calculate the heat of neutralization?
The heat of neutralization can be calculated using the formula Q = mCΔT, where Q represents the heat released or absorbed, m is the mass of the solution, C is the specific heat capacity of the solution, and ΔT is the change in temperature.
What are some potential sources of error in a heat of neutralization experiment?
Potential sources of error include inaccurate temperature measurements, incomplete reactions, heat loss to the surroundings, and impurities in the reactants.