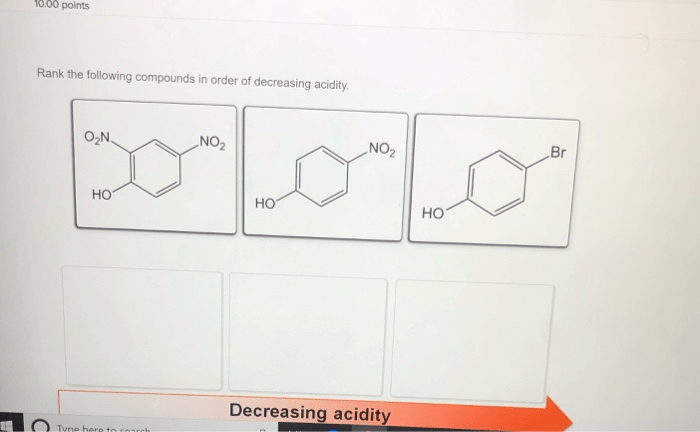
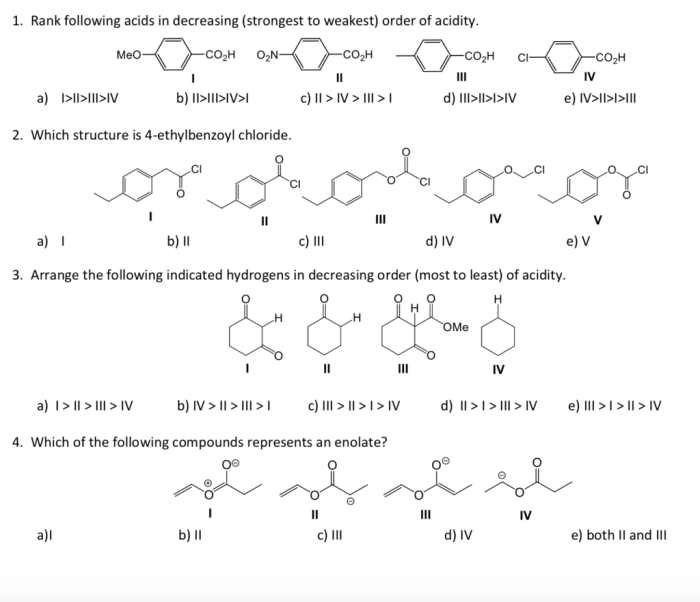
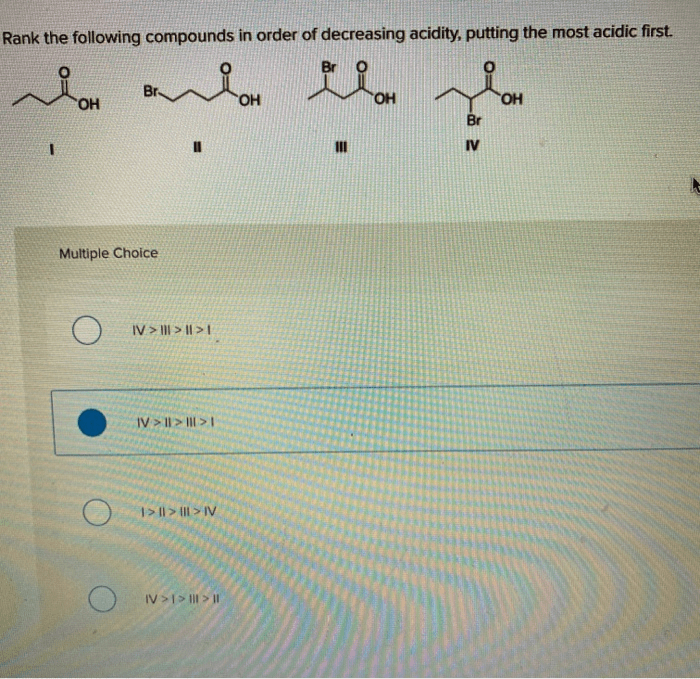
Rank the following acids in order of decreasing acidity – Delving into the realm of acid strength, this comprehensive guide explores the concept of acid strength and its measurement. By examining the factors that influence acid strength, we can understand how to rank acids in order of decreasing acidity. This knowledge has significant applications in various fields, from industry to medicine, highlighting the importance of understanding acid strength in shaping our world.
To provide a clear understanding, we will create a table to rank the following acids in order of decreasing acidity: hydrochloric acid (HCl), sulfuric acid (H2SO4), nitric acid (HNO3), and acetic acid (CH3COOH). We will delve into the role of electronegativity and molecular structure in determining acid strength, providing examples to illustrate these concepts.
Acid Strength and Properties
Acidity is a measure of the ability of a substance to donate protons (H+ ions). The stronger the acid, the more readily it donates protons. Acid strength is typically measured using the pH scale, which ranges from 0 to 14. A pH of 0 indicates a highly acidic solution, while a pH of 14 indicates a highly basic solution.
Neutral solutions have a pH of 7.
Several factors influence acid strength, including:
- Electronegativity of the atom bonded to hydrogen
- Molecular structure
- Concentration
Ranking Acids in Order of Decreasing Acidity
| Acid | Formula | Acidity |
|---|---|---|
| Hydrochloric acid | HCl | Strong |
| Sulfuric acid | H2SO4 | Strong |
| Nitric acid | HNO3 | Strong |
| Acetic acid | CH3COOH | Weak |
The ranking of acids in order of decreasing acidity is based on their dissociation constants. The dissociation constant is a measure of the extent to which an acid dissociates into ions in solution. The higher the dissociation constant, the stronger the acid.
Factors Affecting Acid Strength
Electronegativity
The electronegativity of the atom bonded to hydrogen plays a crucial role in determining acid strength. Electronegativity is a measure of an atom’s ability to attract electrons. The more electronegative the atom, the more strongly it attracts electrons, making it less likely to donate protons.
For example, hydrogen fluoride (HF) is a weaker acid than hydrogen chloride (HCl) because fluorine is more electronegative than chlorine.
Molecular Structure
Molecular structure also affects acid strength. Acids with a more dispersed charge are generally stronger than those with a concentrated charge. For example, sulfuric acid (H2SO4) is a stronger acid than nitric acid (HNO3) because the charge in sulfuric acid is dispersed over two sulfate ions, while in nitric acid, the charge is concentrated on a single nitrate ion.
Applications of Acid Strength, Rank the following acids in order of decreasing acidity
Acid strength has numerous applications in various fields, including:
- Industry:Acids are used in the production of fertilizers, plastics, dyes, and other chemicals.
- Medicine:Acids are used in the production of pharmaceuticals, as well as in the treatment of certain medical conditions.
- Research:Acids are used in a wide range of scientific research, including the study of chemical reactions and the development of new materials.
Understanding acid strength is essential for optimizing these applications and ensuring their safety and effectiveness.
FAQ Overview: Rank The Following Acids In Order Of Decreasing Acidity
What factors influence acid strength?
Electronegativity, molecular structure, and the presence of resonance structures all play a role in determining acid strength.
Why is it important to rank acids in order of decreasing acidity?
Ranking acids helps us understand their relative strengths and predict their behavior in chemical reactions.
How can acid strength be applied in real-world applications?
Acid strength is essential in industries like manufacturing, medicine, and research, where it influences the effectiveness of chemical processes and treatments.