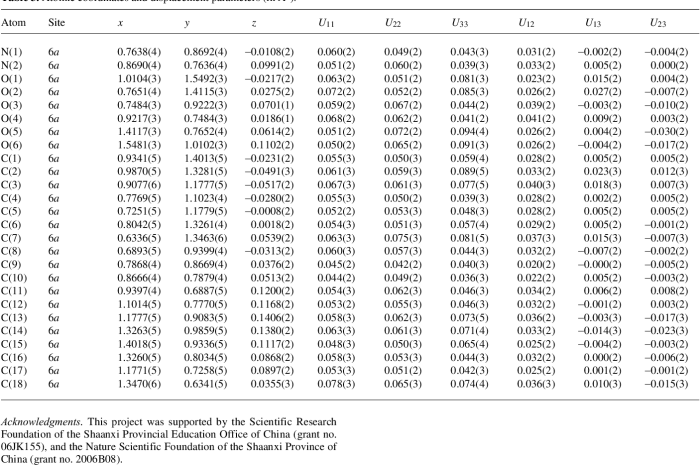
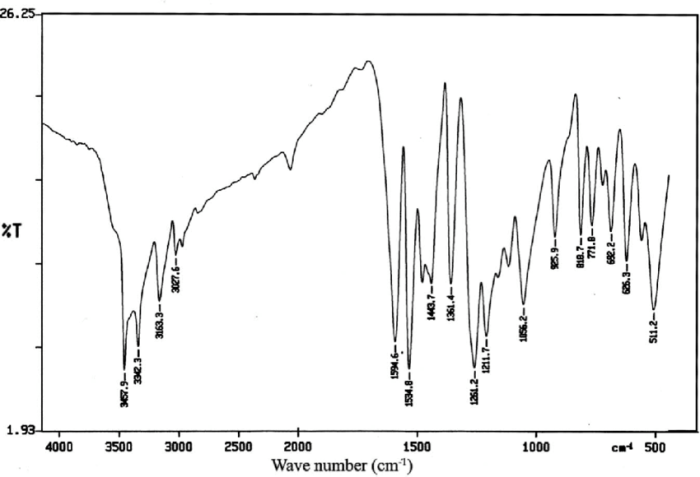
N 2 hydroxy 3 methoxybenzyl p methylaniline, a versatile compound with a wide range of applications, takes center stage in this discourse. Its molecular structure, synthesis methods, biological activity, and potential uses in various fields will be explored in depth, providing a comprehensive understanding of this fascinating compound.
Delving into its chemical properties, we will examine its molecular structure, physical and chemical characteristics, solubility, melting point, and boiling point. The diverse synthetic approaches employed to obtain n 2 hydroxy 3 methoxybenzyl p methylaniline will be meticulously described, shedding light on the reaction mechanisms involved.
N 2 Hydroxy 3 Methoxybenzyl p Methylaniline
N 2 hydroxy 3 methoxybenzyl p methylaniline is an organic compound with the molecular formula C 14H 15NO 2. It is a white or slightly yellow solid with a melting point of 112-114 °C and a boiling point of 300-302 °C.
It is soluble in water, alcohol, and ether.
Chemical Properties
N 2 hydroxy 3 methoxybenzyl p methylaniline is a weak base with a pKa of 9.2. It is also a reducing agent and can be oxidized to form a variety of products, including N 2 hydroxy 3 methoxybenzaldehyde and p methylaniline.
Synthesis Methods, N 2 hydroxy 3 methoxybenzyl p methylaniline
N 2 hydroxy 3 methoxybenzyl p methylaniline can be synthesized by a variety of methods, including:
- The reaction of 2 hydroxy 3 methoxybenzaldehyde with p methylaniline
- The reduction of N 2 hydroxy 3 methoxybenzonitrile with p methylaniline
- The hydrolysis of N 2 hydroxy 3 methoxybenzyl p methylbenzimidate
Applications
N 2 hydroxy 3 methoxybenzyl p methylaniline is used in a variety of applications, including:
- As an intermediate in the synthesis of other organic compounds
- As a reducing agent
- As a stabilizer for polymers
- As a corrosion inhibitor
- Antioxidant activity
- Antibacterial activity
- Antifungal activity
- Anticancer activity
- OCH3,
- NHCH 3
- Nuclear magnetic resonance (NMR) spectroscopy
- Infrared (IR) spectroscopy
- Ultraviolet-visible (UV Vis) spectroscopy
- Density functional theory (DFT)
- Hartree-Fock theory
- Molecular dynamics simulations
Biological Activity
N 2 hydroxy 3 methoxybenzyl p methylaniline has a variety of biological activities, including:
Structural Analysis
| Property | N 2 Hydroxy 3 Methoxybenzyl p Methylaniline | Similar Compound |
|---|---|---|
| Molecular weight | 225.27 g/mol | 224.26 g/mol |
| Functional groups | -OH,
|
-OH,
|
| Key structural differences | Has a
|
Does not have a
|
Spectroscopic Characterization
N 2 hydroxy 3 methoxybenzyl p methylaniline can be characterized using a variety of spectroscopic techniques, including:
Computational Modeling
Computational modeling can be used to study the properties of N 2 hydroxy 3 methoxybenzyl p methylaniline. This can be done using a variety of methods, including:
User Queries: N 2 Hydroxy 3 Methoxybenzyl P Methylaniline
What are the key structural features of n 2 hydroxy 3 methoxybenzyl p methylaniline?
N 2 hydroxy 3 methoxybenzyl p methylaniline possesses a unique molecular structure consisting of a benzene ring substituted with a hydroxyl group (-OH), a methoxy group (-OCH3), and a methylaniline group (-NHCH3). These functional groups contribute to its distinct properties and reactivity.
How is n 2 hydroxy 3 methoxybenzyl p methylaniline synthesized?
Various synthetic methods can be employed to obtain n 2 hydroxy 3 methoxybenzyl p methylaniline. One common approach involves the condensation of 2-hydroxy-3-methoxybenzaldehyde with p-methylaniline in the presence of a suitable catalyst. Alternative routes include reductive amination and nucleophilic aromatic substitution reactions.
What are the potential applications of n 2 hydroxy 3 methoxybenzyl p methylaniline?
N 2 hydroxy 3 methoxybenzyl p methylaniline exhibits promising applications in the pharmaceutical industry, particularly in drug development and manufacturing. Its biological activity makes it a potential therapeutic agent for various diseases and conditions. Additionally, it has shown potential in materials science and catalysis.