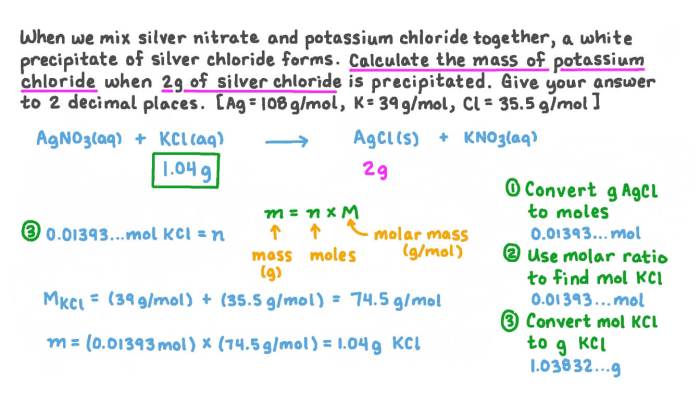
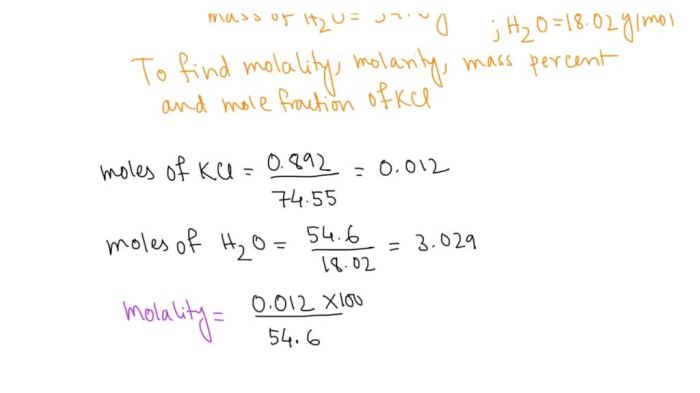Two grams of potassium chloride are completely dissolved, embarking on a captivating journey that unravels the intricate properties, dissolution process, concentration, and diverse applications of this remarkable compound. This in-depth exploration unveils a wealth of knowledge, providing a comprehensive understanding of potassium chloride’s multifaceted nature.
Potassium chloride, a substance of significant importance, possesses a unique chemical formula and molecular weight. Its physical and chemical properties, meticulously examined, reveal its distinctive characteristics. The dissolution process of potassium chloride in water, a fundamental aspect of its behavior, is thoroughly described, shedding light on the factors that influence its rate of dissolution.
Properties of Potassium Chloride: Two Grams Of Potassium Chloride Are Completely Dissolved
Potassium chloride (KCl) is an ionic compound composed of potassium (K+) and chloride (Cl-) ions. It has a chemical formula of KCl and a molecular weight of 74.55 g/mol.Physically, potassium chloride is a white crystalline solid with a density of 1.98 g/cm3.
It is soluble in water, forming a colorless solution. Chemically, potassium chloride is a strong electrolyte, meaning it dissociates completely into its ions in water.
Dissolution of Potassium Chloride
When potassium chloride is added to water, it undergoes a dissolution process. During dissolution, the water molecules surround and interact with the potassium and chloride ions, breaking the ionic bonds and allowing the ions to disperse into the solution.The rate of dissolution is affected by several factors, including:
- Temperature: Higher temperatures increase the kinetic energy of the water molecules, making them more effective at breaking the ionic bonds and increasing the rate of dissolution.
- Surface area: Smaller particles have a larger surface area, allowing more water molecules to interact with the potassium chloride and increasing the rate of dissolution.
- Agitation: Stirring or shaking the solution helps to disperse the potassium chloride particles and increases the rate of dissolution.
Concentration of Potassium Chloride Solution, Two grams of potassium chloride are completely dissolved
The concentration of a potassium chloride solution can be expressed in terms of molarity (M), which is the number of moles of potassium chloride dissolved in one liter of solution. To calculate the concentration of a potassium chloride solution prepared by dissolving two grams of potassium chloride in a specific volume of water, we can use the following formula:“`Concentration (M) = Moles of potassium chloride / Volume of solution (L)“`First, we need to convert the mass of potassium chloride to moles using its molar mass:“`Moles of potassium chloride = Mass / Molar mass = 2 g / 74.55 g/mol = 0.0268 mol“`Assuming the volume of the solution is 100 mL (0.1 L), the concentration of the potassium chloride solution would be:“`Concentration (M) = 0.0268 mol / 0.1 L = 0.268 M“`
Applications of Potassium Chloride Solution
Potassium chloride solution has various applications, including:
- Medicine:Potassium chloride is used as an electrolyte supplement to treat low potassium levels in the blood (hypokalemia). It can also be used to treat cardiac arrhythmias and hypertension.
- Agriculture:Potassium chloride is a common fertilizer used to supplement potassium in soil, which is essential for plant growth.
- Industry:Potassium chloride is used in the production of glass, ceramics, and textiles. It is also used as a flux in soldering and welding.
Frequently Asked Questions
What is the chemical formula of potassium chloride?
Potassium chloride’s chemical formula is KCl.
What is the molecular weight of potassium chloride?
The molecular weight of potassium chloride is 74.55 g/mol.
What factors affect the rate of dissolution of potassium chloride?
Factors affecting the rate of dissolution include temperature, surface area, agitation, and solvent polarity.